- About Us
- Our Clients
- Services
- Insights
- Healthcare Sectors
- Ambulatory Surgery Centers
- Behavioral Health
- Dialysis
- Hospital-Based Medicine
- Hospitals
- Imaging & Radiology
- Laboratories
- Medical Device & Life Sciences
- Medical Transport
- Oncology
- Pharmacy
- Physician Practices
- Post-Acute Care
- Risk-Bearing Organizations & Health Plans
- Telehealth & Healthcare IT
- Urgent Care & Free Standing EDs
- Careers
- Contact Us
The Vertical Consolidation of Tech-Enabled Primary Care Practices
October 27, 2022
Written by Tyler Perper and Madeline Noble
Since the beginning of 2022, the healthcare industry has seen the fast-paced consolidation of tech-enabled primary care practices reach new heights as market participants prepare for a potential shift in the way payments are made for healthcare services. There are a multitude of players seeking to capitalize on the shift from the fee-for-service payment structure to the value-based care (VBC) structure. The fee-for-service payment structure focuses more on the volume of care provided to a risk-bearing, but in the VBC structure, the provider’s services are compensated based on the quality of the care. The current players in the VBC space span a wide range of verticals including payers, health systems, retailers (CVS Pharmacy, Amazon.com, etc.), Medicare Advantage-focused primary care providers (CareMax, Cano Health, etc.), and investors (private equity groups). In this article, VMG Health highlights three transactions we believe illustrate the current state of the VBC space and where it is headed.
Optum Health, a subsidiary of UnitedHealth Group, is considered the market leader in the shift to the VBC payment structure. Optum currently employs over 60,000 physicians at over 2,000 locations. As Optum has expanded its service capabilities, it has focused on data as the key to its success. To that end, in January 2021 UnitedHealth announced the potential acquisition of Change Healthcare for which they would pay approximately $13 billion for the business.
Although the Department of Justice filed an antitrust lawsuit against the acquisition, a Washington federal judge denied the DOJ’s attempt to block the deal in September. As a result, Change Healthcare will join OptumInsight, the data and analytics division of Optum Health, to provide software and data analytics, technology-enabled services, and research, advisory and revenue-cycle management offerings (1). The combined entity will bring together the two largest electronic data clearinghouses which will provide Optum Health with crucial data on how to optimize their patient care service (2). Though the current deal is under review by the DOJ for anti-trust reasons, the investment by Optum Health maintains the intentions of the market leader in VBC by investing big in its data and analytics arm.
Per the president of UnitedHealth Group and the CEO of Optum Health, “Together (Optum Health and Change Healthcare) we will help streamline and inform the vital clinical, administrative, and payment processes on which healthcare providers and payers depend to serve patients.” (1)
To maximize the value-based care payment model, the players in the space have realized the importance of patient data to enhance patient care and lower the cost of managing a patient population. In addition, UHC’s acquisition of Change Healthcare is another example of payers looking to diversify their capabilities to capture the entire flow of the premium dollar (3).
Another monumental value-based care deal in 2022 was CareMax acquiring Steward Health Care System’s Medicare Advantage business. As part of the deal with Steward Health, CareMax became the exclusive management service organization for Steward’s Medicare value-based care business which includes 171,000 lives (50,000 MA lives, 112,000 MSSP lives, and 9,000 direct contracting lives) across eight states in the Southeast of the U.S. (4).
CareMax, another organization that understands the value of data and analytics, is a leading technology-enabled provider of value-based care to seniors. CareMax, Cano Health, Oak Street Health, and other full-risk primary care clinics are partnering with payors to ensure the patient’s care is provided in the correct setting, focuses on preventative medicine, and ultimately, lowers the mortality rates (especially among older populations) (5). CareMax’s focus on whole person health through a unique blend of targeted technology and comprehensive, high-touch care has resulted in improved clinical outcomes compared to peers and traditional Medicare benchmarks. CareMax has achieved a five-star quality rating, the highest possible rating, across its locations overall (6). The CareMax/Steward Health deal shows the continued growth trajectory of the tech-enabled PCP business model and how providers with the proper insights are willing to bear additional risk on behalf of their patient population.
Key Takeaways
- Competing on value and population-based risk (through COVID-based learnings) has renewed interest among traditional channels (providers and payers) in addition to other newer entrants (Optum, Amazon, etc.).
- Market entrants are moving aggressively on a different strategic course. In turn, risk-bearing entities (outside of traditional health system players) will take on more directed roles in acquiring patient lives with traditional provider organizations losing premium dollar market share.
- Traditional provider organizations (hospitals and health systems) require a different strategic lens to view stratified patient populations if they want to compete in the risk-based sector.
- Data continues to suggest patients have higher overall affinity to their doctor and supporting hospital versus payers or payer-related services. However, with significant, generalized inflationary pressures consumerism will further push patients and consumers to evaluate non-traditional programs and plan types.
- Technology platforms are being built to large scale and at a faster rate than any local healthcare provider can do alone. Providers may need to consider joining with a technological partner to compete more aggressively when considering acceptance of insurance risk.
Lastly, in July Amazon made its entry into the primary care and the VBC space through its $3.9 billion acquisition of One Medical, a membership-based, tech-enabled provider of primary care. Back in June 2021, One Medical announced a $2.1 billion deal with Iora Health, a VBC care PCP clinic focused on Medicare and Medicare Advantage patients. This deal expanded One Medical’s patient population from solely commercially insured patients to include Medicare patients and introduce risk-based care models. One Medical’s strong clinical growth, with a 180+ current clinic footprint, and new VBC models require large capital spending. Due to this, One Medical had an operating loss of $255 million in 2021 (7).
Questions remain regarding Amazon’s plans with One Medical, but one thing is for certain: Amazon has the analytical capability, customer base, and capital needs to turn One Medical into a cash flow machine. Additionally, Amazon has the capacity to take on enormous amounts of risk which is needed to successfully run Medicare and Medicare Advantage risk-based care models as a part of One Medical’s Iora segment. Amazon can transform the delivery of healthcare services by making it more accessible and affordable for the millions of customers they reach. There are many opportunities for integration with existing Amazon services, including Amazon Care and the Amazon Prime subscription service, which could expand One Medical’s current capabilities. This transaction, along with many others, might be the beginning of Big Tech in the VBC space.
The rapid vertical consolidation of tech-enabled primary care organizations is representative of the opportunities noticed by existing and new players. It is an opportunity to transform the primary care delivery into risk-bearing, population-specific models focused on lowering healthcare costs of the patient population while tailoring offerings specific to patient needs.
Providers should be prepared to transform their care delivery for the potential evolution to the VBC payment structure by retooling financial and clinical strategies, and by converting patient population data into actionable information. Data and analytics are the key for providers to achieve high value care for all stakeholders. To ensure that your primary care clinic is set up for success under the VBC delivery of care model you need to have a contracted network of specialists and medical centers, the proper assortment of non-physician practitioners to treat patients, and an electronic health record system in place that can more seamlessly facilitate care coordination and the flow of patient information across clinical sites.
Sources & Endnotes:
- https://www.healthcareitnews.com/news/optum-acquire-change-healthcare-13b-deal
- https://www.bloomberg.com/news/articles/2022-08-01/unitedhealth-unh-change-healthcare-chng-deal-doj-challenge-gets-underway
- https://workweek.com/2022/08/01/3m-and-labcorp-spinoff-healthcare-news-8-2-2022/
- https://workweek.com/2022/06/07/steward-health-care-value-based-deal-caremax/?utm_campaign=%5Bcampaign_name%5D&utm_medium=email&utm_source=Sailthru&utm_term=Hospitalogy Jeffries – “HC Payment Care Delivery Landscape Evolving”
- Jeffries Research Services, LLC. (October 20, 2021). H/C Payment/Care Delivery Landscape Evolving as Value-Based Models Gain Ground. Jefferies LLC.
- https://www.businesswire.com/news/home/20220601005579/en/CareMax-Inc.-to-Acquire-Medicare-Value-Based-Care-Business-of-Steward-Health-Care-System
- https://www.washingtonpost.com/business/2022/07/22/amazon-one-medical-faq/
- https://investor.onemedical.com/news-releases/news-release-details/one-medical-announces-agreement-acquire-iora-health
- https://www.fiercehealthcare.com/health-tech/amazon-shells-out-39b-primary-care-startup-one-medical
- https://www.mdclarity.com/preparing-providers-for-value-based-care/
Categories: Uncategorized
Physician Practice Strategy: The Private Equity Play
October 20, 2022
Written by Holden Godat and Taylor Anderson, CVA
Health systems, outpatient facilities, payors, and private equity firms (PE) are all vying for alignment with physicians since they drive practically all healthcare decisions. As a result, physicians are faced with many choices regarding employment and strategic partnerships. Of all these choices, the PE play appears to have grown the most among physician practices. The following outlines fundamentals to the PE option, as well as how health system partners offer different opportunities.
The Evolution of Private Equity
Private equity (PE) investment in healthcare is not particularly new. However, the level of PE investment in healthcare has climbed from $19.5 billion in 2015 to $74.4 billion in 2022. (1) The growth of PE activity is also not projected to slow down anytime soon as the total number of deals has exceeded 1,000 in each of the past three years. (2) With projected increases in healthcare spending, along with significant stores of uninvested capital, PE investment in healthcare is still anticipated to flourish in the coming years. (1)
Over time, PE has expanded its area of focus from a few specialties to a much broader range of specialties. VMG Health has observed that there was a time when PE investment was focused primarily on specialties such as anesthesiology, dermatology, and gastroenterology. (3) Today, VMG Health has observed that significant PE investment is now focused on a wider range of specialties, such as primary care, urgent care, ophthalmology, and dentistry. (4) Additionally, the pandemic brought about new opportunities for PE investment including, but not limited to, behavioral and mental health as well as telehealth. (5)
Even with the growth of PE activity, health systems still maintain a significant advantage over the PE model- the advantage of specialty mix. PE firms typically have the limitation of focusing on a single specialty or at most a few similar specialties. Health systems, on the other hand, have the ability and infrastructure to integrate across a broad spectrum of specialties, which can lead to a larger referral stream for affiliated physicians. This larger referral stream also carries the secondary benefit of a higher quality of patient care by providing patients with a more connected continuum of care, which is a significant theme in healthcare today.
Regulatory Landscape
PE investment in healthcare is not as straightforward as PE investment in other industries. In most other industries, a PE firm could simply utilize its capital to purchase a controlling stake in a business. However, the financially driven nature of PE has the potential to negatively impact the quality of patient care. This makes PE investment into healthcare a bit more complex by adding regulatory hurdles such as the Corporate Practice of Medicine (CPOM) Doctrine.
The CPOM Doctrine was developed to protect the quality of patient care. It is described by the American Medical Association (AMA) as a doctrine that “prohibits corporations from practicing medicine or employing a physician to provide professional medical services.” (6) The CPOM Doctrine in individual states may vary slightly; therefore, it is very important that the PE firm is aware of the specific state level adaptations that could impact a potential transaction or investment.
In addition to CPOM, there are additional regulatory factors that could impact the growth of PE activity in the healthcare space. One such event is a recent executive order that was issued by President Joe Biden in July 2021 that will likely cause further scrutiny of PE activity in the healthcare space. (5) Further, there have been instances where PE firms were held liable and were forced to pay damages for wrongdoing perpetrated by a healthcare entity related to the PE firm, even though the PE firm itself was not found to have perpetrated any wrongdoing. (7) Lastly, there is a growing focus by the Department of Justice and Federal Trade Commission to focus on anti-trust enforcement in private equity related ventures. (8) The regulatory scrutiny found in the healthcare space is somewhat unique and certainly provides a new challenge for the typical PE firm.
Unlike PE firms, health systems have long been aware of the regulatory environment surrounding healthcare. This is an advantage to a physician practice since health system leaders are typically apprised of regulatory risks and understand how to protect the parties from scrutiny. Many healthcare regulations, such as CPOM, require value exchanged with physicians to be set at fair market value. It is important for physicians to understand this standard is applicable in most transactions whether it be a health system or PE company.
The MSO Structure
Even with the presence of the CPOM Doctrine, many PE firms are still investing in the healthcare industry through the creation of a Management Services Organization (MSO). Once a PE firm creates an MSO, that MSO will typically enter into a comprehensive management services agreement (MSA) with a physician practice. Through the MSA, the MSO will typically provide as many services allowed under the state’s CPOM Doctrine. These services may include, but are not limited to, the provision of accounting, billing and collection, non-clinical personnel, supply procurement, equipment, office space, and numerous other services. In exchange for the provision of the subject services the physician practice will compensate the MSO through a management fee. This management fee is what effectively serves as the return for the PE firm’s investment and should be set at fair market value.
The MSO model provides numerous benefits for the physicians. The first benefit is the physicians receive a lump sum amount upfront which carries a high multiple on the physician practice’s earnings. This lump sum comes in exchange for the physicians’ agreement to lower levels of compensation moving forward. Many PE healthcare transactions also involve the physicians receiving “roll-over” equity in the MSO. This “roll-over” equity provides the physicians with some future compensation upside, and serves to keep the physicians motivated to maintain (and/or grow) the clinical operations of the practice. In addition to the monetary benefits the physicians receive from the MSO model, they also retain physician control and governance over the clinical operations of their practice.
A major strategic difference between an MSO and partnering with a health system is PE firms are typically eyeing shorter and more defined time horizons between transactions. On the other hand, health systems often have longer strategic time horizons which allow them to employ physicians for longer terms. This ability to align with a health system for the long term can be very beneficial for a physician seeking stability. Additionally, health systems have the ability to implement unique compensation models that can better support physician motivations.
Private Equity vs. Health System Take-Aways
It is obvious physician practices are in high demand and have numerous choices for partnerships in the market. Due to the fragmentation that exists in the healthcare space, the significant stores of uninvested capital, and the growth of new healthcare services, PE activity in the healthcare space is not projected to slow anytime soon. PE firms have brought an innovative model to the healthcare space that many practices are considering. However, physician practices should consider the differences between the MSO model and health system opportunities prior to pursuing a strategy.
Sources & Endnotes:
- Sourced from Pitchbook, Inc. data
- Sourced from Pitchbook, Inc. data
- VMG Health observed these specialties being the most common specialty for PE investment for several years.
- Based on observations of common specialties in the valuations the authors have done.
- Sourced from the article titled “Private Equity and Health Care Investments: How Has COVID-19 Impacted Deal Flow?” that was published by the Transaction Affinity Group of AHLA’s Business Law and Governance Practice Group on September 15, 2021.
- Sourced from file:///C:/Users/Taylor.Anderson/Downloads/corporate-practice-of-medicine-issue-brief_1.pdf
- Sourced from https://www.reuters.com/legal/government/private-equity-firm-hig-capital-settles-fraud-case-20-million-2021-10-14/
- Sourced from remarks made by Andrew J. Forman at the American Bar Association’s Antitrust in Healthcare Conference Keynote Address, June 3, 2022.
Categories: Uncategorized
Laboratory-Developed Tests Save the Day!
October 3, 2022
Written by Mason Motal, Aaron Murski, CVA, Austin Grawe, and Patrick Speights
The coronavirus disease 2019 (COVID-19) is caused by the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was first discovered in December 2019 in Wuhan, China. COVID-19 at the time was a new human virus causing respiratory illness and was very contagious, quickly spreading from person to person. Within a few months it was announced by the CDC as a global pandemic on March 11, 2020. The laboratory industry began leading at the forefront to combat the virus, and faced many unique, complex challenges. Hundreds of thousands of people in the United States were getting swabbed every day for COVID-19, and this caused labs to face severe supply shortages and for staffs to be overwhelmed with more tests than they could handle. Laboratory employees were working 24/7 around-the-clock to try to keep up with the high testing demand. Still, they were running out of essential products faster than manufacturers could restock them.
“If the demand keeps increasing like it’s been increasing over the last couple of weeks, the lab industry will never be able to keep up with it,” said Dr. Dwayne Breining, executive director of Northwell Health Laboratories. “I think the top priority is going to be mitigating the clinical spread of the virus by doing things we know work: things like social distancing, masking, and monitoring. That would allow you to slow it down enough so that not only the lab testing industry, but the entire medical system can catch up.” (6) (10)
Clinical Laboratory Improvement Amendments Overview
The Clinical Laboratory Improvement Amendments (CLIA) was established in 1988 to strengthen federal oversight of clinical laboratories and prohibit them from testing human specimens for diagnosis, prevention, treatment, or health assessment without a valid CLIA certificate. To become CLIA-certified labs must establish specific performance characteristics such as accuracy, quality, and reliability as imposed by the CLIA statute. According to the CDC, approximately 14 billion lab tests are ordered annually, and roughly 70 percent of today’s medical decisions are based on lab results. Therefore, CLIA ensures that patients and health care providers receive accurate, dependable test results.
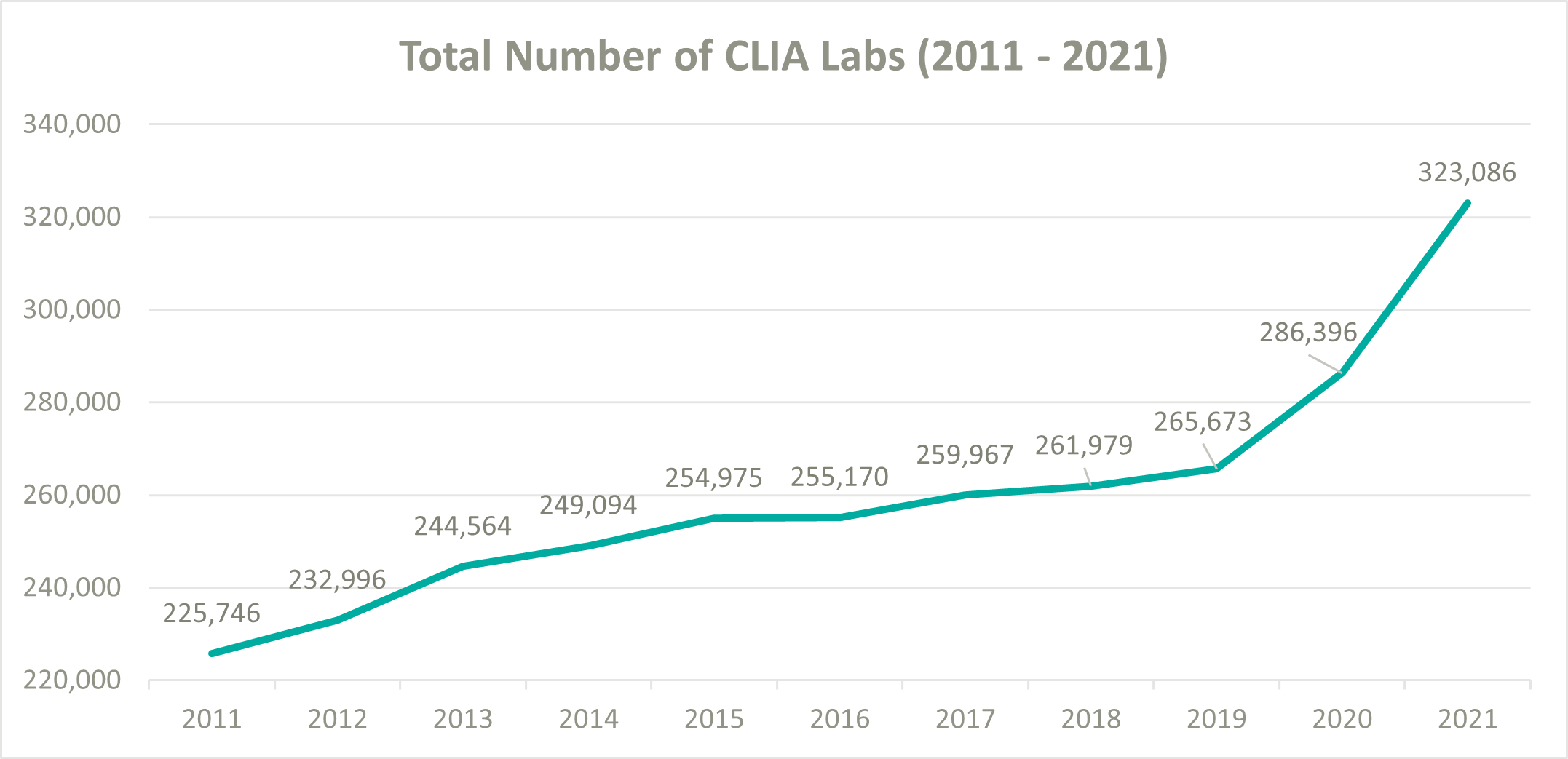
To help ease pressure on the lab industry during the onset of COVID-19, the Centers for Medicare and Medicaid Services (CMS) decided to loosen their CLIA policies and allowed laboratories wishing to perform COVID-19 tests to become CLIA certified and begin testing patients as quickly as possible. According to the CMS database, the total number of CLIA labs increased from 265,673 at the beginning of 2020 to 323,086 by the end of 2021, resulting in a 10.3% compound annual growth rate (CAGR). In comparison, from 2011 to 2019 the number of CLIA labs only grew from 225,746 to 265,673, a 2.1% CAGR. (5) (1) (7)
Laboratory-Developed Tests Overview
Typically, laboratory tests are developed by diagnostics manufacturers that produce test kits to sell to clinical labs. However, tests are also developed by labs that manufacture and use them in their own facilities, known as laboratory-developed tests (LDTs). LDTs are defined by the Food and Drug Administration (FDA) as an in vitro (in glass) diagnostic test that is manufactured by and used within a single laboratory. LDTs are commonly referred to as “in-house” or “home brew” tests. The FDA regulates the manufacturing of test kits by diagnostics manufacturers under its medical device authorities. Still, it exercises regulatory discretion to allow labs to use their LDTs pursuant to rules established under CLIA. CLIA’s analytical validation is limited to specific conditions, staff, equipment, and patient populations. Therefore, LDTs are reviewed during the routine biennial survey and are constantly monitored after the testing begins.
Furthermore, CLIA requirements are based on test complexity, therefore labs that develop LDTs must meet stricter requirements than standard labs. In contrast, the FDA’s premarket clearance and approval processes assess the analytical validity of a test system in much greater depth and scope. The FDA’s review of analytical validity is done prior to the marketing of the test system, as well as assessing clinical validity, which is part of the review focused on the safety and effectiveness of the test system. The CLIA and FDA regulatory systems differ in levels of focus, scope, and purpose; however, they are intended to be complementary to each other. (1) (2) (13)
LDTs Impacting the Lab Industry
When COVID-19 initially hit the U.S. in March 2020, the CDC was unable to provide testing kits for the first several weeks due to the contamination of tests at a manufacturing lab. Therefore, the government decided to permit diagnostic manufacturers to develop COVID-19 test kits and sell them to labs. They were also permitted to sell them to independent and hospital labs to develop LDTs under emergency use authorizations (EUAs). The primary benefit of an LDT over a typical diagnostic test is the time involved in the approval process. A routine diagnostic test can take years to pass through the many phases of research, testing, clinical evaluation, development of manufacturing processes, and review by the FDA or other regulatory authorities before a commercial test is available for public use. Whereas LDTs are designed to take only a few months to pass clearance and become regulated for use.
By late July 2020, LabCorp was processing nearly 180,000 tests for COVID-19 per day with an average turnaround time of three to five days. At the same time, Quest Diagnostics was performing 135,000 tests daily with an average wait of at least seven days.
President, CEO, and Chairman of LabCorp Adam H. Schechter, noted during their Q2 2020 earnings call how vital LDTs were in helping them keep up with the high demand for tests, “We launched back in March with our LDT, and it’s been remarkable the amount of volume we’ve been able to do through that LDT. We were doing 2,000 to 3,000 PCR tests per week, and here we are at the end of July, and we’re able to do 180,000 PCR tests per day. Of course, we still need our suppliers and the other companies to help us get to 180,000, but I have to say that our scientists did an extraordinary job with the LDT to get us to 180,000 today.”
When asked how many of the 180,000 tests were related to LDTs, Schechter said, “I’m not going to give you the exact number through the LDT because that changes, but we have 16 labs running tests. So, at the end of the day, it depends on where the samples are, which tests we run them, and what we try to do is optimize our network so we can get the best possible turnaround we can.”
Mark J. Guinan, VP, and CFO of Quest Diagnostics, also spoke on the positive impact of LDTs during their Q2 2020 earnings call.
“We exited the second quarter averaging approximately 110,000 and 26,000 COVID-19 molecular and serology tests, respectively, each day,” he said. “Over the next couple of weeks, we expect to have the capacity to perform 150,000 molecular diagnostic tests a day. We are providing test results in about two days for the highest priority patients. And the average turnaround time for nonpriority patients is at least seven days.”
Comparatively, before the pandemic the typical turnaround time for a lab test was one to two days.
LDTs Regulatory Landscape Continued
On August 19, 2020, the Department of Health and Human Services (HHS) Secretary Alex Azar announced HHS was rescinding all informal policy documents issued by the FDA related to LDTs because the FDA had not engaged in “notice-and-comment” rulemaking. The FDA had not required labs developing LDTs to submit tests for premarket review and had deprioritized the review of EUA requests for COVID-19 LDTs in favor of traditional, kit-based in vitro diagnostics (IVDs) from commercial manufacturers. As a result, such products were no longer required to secure a EUA or other non-emergency marketing authorization from the agency before being launched commercially. However, labs that chose to use LDTs without FDA premarket review or authorization would no longer be eligible for Public Readiness and Emergency Preparedness (PREP) Act coverage absent approval, clearance, or authorization and they would remain subject to regulation under CLIA.
According to HHS.gov, the PREP Act authorizes the secretary of the HHS to issue a declaration providing immunity from liability for claims; 1) of loss caused, arising out of, relating to, or resulting from administration or use of countermeasures to diseases, threats, and conditions, 2) determined by the secretary to constitute a present, or credible risk of a future public health emergency, 3) to entities and individuals involved in the development, manufacturing, testing, distribution, administration, and use of such countermeasures. Labs that already had an active EUA for LDTs were unaffected by the announcement.
With the pandemic appearing to slow down and commercial tests for COVID-19 becoming abundant, HHS hit the reset button for the regulation of LDTs on November 15, 2021. The FDA once again had regulatory authority and required clinical labs to submit EUA requests to continue performing and marketing such tests. The agency reported in their November 15 press release that the current areas of focus for EUA reviews were: 1) at-home and point-of-care (POC) diagnostic tests, 2) certain high-volume, lab-based molecular diagnostic tests, 3) certain lab-based and POC high-volume serology tests, and 4) tests submitted or supported by a U.S. government stakeholder.
The dilemma of the FDA’s authority to regulate LDTs has long been a complex and controversial topic for many decades. As mentioned previously, the FDA has historically delegated the oversight of LDTs to CLIA because they initially were relatively simple lab tests and available on a limited basis. However, due to technological advances, LDTs have evolved and are now much more complex with a nationwide reach and present higher risks. The FDA’s main argument is inaccurate test results could lead to people initiating unnecessary treatment, delaying treatment, or foregoing treatment for a health condition that could result in severe illness or death. Whereas most clinical labs feel that LDTs are already sufficiently regulated under CLIA and should not be double regulated under the FDA.
Professor of Pathology and Immunology at Washington University Dennis Dietzen, Ph.D., said, “LDTs fill a void where there is no FDA-approved test. We need to be able to build these things with a healthy amount of regulation, but not a burdensome amount. If labs had to go through premarket review for all their LDTs, it would make the assays more expensive to run, and I think a lot of laboratories would just throw in the towel.” (9) (13) (14) (15) (16) (17) (18) (19)
Conclusion
When COVID-19 initially hit the U.S. in March 2020, the CDC was unable to provide testing kits for the first several weeks due to the contamination of tests at a manufacturing lab. Therefore, the government decided to permit diagnostic manufacturers to develop COVID-19 test kits and sell them to labs. They were also permitted to sell them to independent and hospital labs to develop LDTs under emergency use authorizations (EUAs). The primary benefit of an LDT over a typical diagnostic test is the time involved in the approval process. A routine diagnostic test can take years to pass through the many phases of research, testing, clinical evaluation, development of manufacturing processes, and review by the FDA or other regulatory authorities before a commercial test is available for public use. Whereas LDTs are designed to take only a few months to pass clearance and become regulated for use.
By late July 2020, LabCorp was processing nearly 180,000 tests for COVID-19 per day with an average turnaround time of three to five days. At the same time, Quest Diagnostics was performing 135,000 tests daily with an average wait of at least seven days.
President, CEO, and Chairman of LabCorp Adam H. Schechter, noted during their Q2 2020 earnings call how vital LDTs were in helping them keep up with the high demand for tests, “We launched back in March with our LDT, and it’s been remarkable the amount of volume we’ve been able to do through that LDT. We were doing 2,000 to 3,000 PCR tests per week, and here we are at the end of July, and we’re able to do 180,000 PCR tests per day. Of course, we still need our suppliers and the other companies to help us get to 180,000, but I have to say that our scientists did an extraordinary job with the LDT to get us to 180,000 today.”
Sources:
- https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA
- Laboratory-Developed Tests (LDTs) – Testing.com
- Putting New Laboratory Tests into Practice – Testing.com
- https://www.fda.gov/medical-devices/ivd-regulatory-assistance/clinical-laboratory-improvement-amendments-clia
- QSO-20-21-CLIA (cms.gov)
- Impact of COVID-19 Pandemic on Laboratory Utilization – PubMed (nih.gov)
- Strengthening Clinical Laboratories | CDC
- CDC COVID Data Tracker: Home
- About Face: Laboratory-Developed Tests for COVID-19 Now Subject to EUA Requirements | Mintz
- ‘The challenges that labs are facing are complex’: Why COVID-19 test results are so delayed (nbcnews.com)
- Laboratory Corporation of America Holdings, Q2 2020 Earnings Call, Jul 28, 2020.pdf
- Quest Diagnostics Incorporated, Q2 2020 Earnings Call, Jul 23, 2020.pdf
- COVID-19 and Lab Testing: What’s the Story Behind the Story? | Mintz
- HHS Reverses Course on LDTs: COVID-19 LDTs Again Require FDA Premarket Review | Ropes & Gray LLP (ropesgray.com)
- Laboratory Developed Tests | FDA
- HHS Prohibits FDA from Requiring Premarket Review of LDTs, Including During the COVID-19 Emergency | Ropes & Gray LLP (ropesgray.com)
- Rescission of Guidances and Other Informal Issuances | HHS.gov (archive.org)
- Public Readiness and Emergency Preparedness Act (hhs.gov)
- Back to the Future on Laboratory Developed Tests | AACC.org
- Future of LDT Regulation Hangs in the Balance | AACC.org
Categories: Uncategorized
Where Are My Earnings? Quality of Earnings Analysis in Post-COVID-19 Urgent Care Transactions
August 16, 2022
Written by Melissa Hoelting, CPA
The COVID-19 pandemic sent shockwaves throughout the urgent care industry and fundamentally changed the business of urgent care centers (UCCs). At the start of the pandemic, UCCs had to quickly adapt to offer a new service line and meet the high demand for COVID tests. As the pandemic has progressed and evolved, UCCs have had to continue to adapt to address the effects of vaccination, variants, and at-home testing to their volumes. In addition to this, they have had to adjust to the changing landscape around reimbursement in regard to government mandates on COVID testing and an increase in new patients. As the pandemic has evolved, the VMG Health Quality of Earnings team has been involved in urgent care transactions and has worked firsthand with our clients to quantify the effect of these changes on EBITDA.
COVID-19 History at Urgent Care Centers
Urgent care centers were at the forefront of treatment and testing services throughout the COVID-19 pandemic. After initial volume disruptions during the early onset of the pandemic, urgent care centers experienced significant increases in testing volumes and new patient visits. Average patient visits per clinic (“APVC”) reached record highs during the summer of 2020 when UCCs ramped up testing capabilities and adopted telehealth services. Overall, UCCs with COVID-19 testing capabilities retained more volume than those without testing capabilities.
In addition to the increased visit volumes, the patient mix between new and established patients shifted. Prior to April 2020, patient mix between established and new visits averaged approximately 60% established and 40% new patients. Following COVID spikes, new patient visits became a greater ratio of total visits and represented over 50% of patient visit mix after April 2020 and into 2021. This mix shift had an impact on reimbursement for many urgent care centers. Clinics saw an increase of 52.0% in average net revenue per visit in 2020 as new patients were reimbursed at a higher rate than established patients. This was partially offset by a rise in “no-visit” patients where an E&M code was not billed (e.g., only testing services performed).
During January 2022, through funding from the American Rescue Plan, at-home COVID testing kits were offered online for free. As of May 2022, over 70 million households have visited COVIDTests.gov to order free at-home tests and 350 million tests have been delivered through the program. The program has offered several rounds of free at-home testing mailed through USPS, and eight additional tests were approved for distribution as of May 17, 2022. In addition, pharmacies, online stores, (e.g., Amazon), and retail locations have expanded the accessibility of at-home testing kits.

Volume-Related Quality of Earnings Adjustments
To quantify the impact of these trends on an urgent care center’s quality of earnings, we must first distinguish between the three types of visits – asymptomatic COVID visit, symptomatic COVID visit, and traditional urgent care visit. Many UCCs now require an office visit whenever a COVID test is administered, therefore we cannot solely use CPT codes to separate between asymptomatic and symptomatic visits but instead must rely on the ICD-11 codes. Based on ICD-11 codes, asymptomatic COVID visits include patients who only have a COVID test code and, in some cases, a comorbidity code such as having high blood pressure, being a smoker, etc. Symptomatic COVID visits include both a COVID test code and symptom codes (i.e., cough, sneeze, sore throat, etc.). Finally, traditional UCC visits include all remaining visits. Once these visits have been categorized we can begin adjusting to expected go-forward volumes.
First, we evaluate the appropriate run-rate of asymptomatic COVID testing. At the start of the pandemic, few individuals received an asymptomatic COVID test since facilities often experienced shortages of tests and chose to prioritize symptomatic patients. In 2021, asymptomatic testing increased as individuals sought testing to meet requirements imposed for travel, employment, and other reasons. Recently, asymptomatic testing demand has fallen (decreased?) as many cities, states, and countries have either eliminated testing requirements or replaced them with vaccine requirements. Due to the changing landscape of asymptomatic testing, we use a narrow time frame of three to six months to determine the appropriate run-rate volume.
Next, we determine the go-forward state of symptomatic COVID testing. Before the prevalence of vaccines, symptomatic COVID testing dominated UCC volume. At this time, the only downward pressure on volume came from supply shortages. After widespread availability of vaccines in early 2021, UCCs began to experience periods of both high and low demand for symptomatic testing as new variants began to emerge. In addition to this more seasonal nature of demand, at-home testing became more prevalent, and was further accelerated by the funding from the American Rescue Plan in January 2022. Based on all these factors, we use a look back period of eight to 12 months for calculating average go-forward volume. This timeframe ensures we capture seasonality while only including months with similar circumstances regarding vaccinations and at-home testing.
Finally, we evaluate the expected recovery of traditional urgent care volume. During the period of stay-at-home orders in 2020, UCCs saw a large fall off in non-testing volume. This was especially prevalent as many facilities temporarily became COVID-only sites. After stay-at-home orders were lifted, facilities still saw lower non-testing volume due to other restrictions put in place such as mask mandates and social distancing. These restrictions resulted in a milder cold and flu season at the time. As traditional volumes remain depressed, 2019 represents the ideal benchmark for estimating future urgent care volume as it is the last year untouched by COVID.
However, relying on 2019 volumes to determine a new run-rate comes with its own challenges stemming from capacity constraints, hiring challenges, and volume trends. In 2019, most UCCs were ideally operating close to full capacity with only traditional visits. Thus, we need to balance expected COVID test volume with a return to normal to ensure we do not project total monthly volume beyond the capacity of a location. Additionally, many UCCs have reported hiring challenges due to the shortage of nurses and mid-level providers which make up the bulk of staff for most facilities. We address this challenge to our calculation by holding conversations with management to understand the specific facility’s hiring challenges and to determine if traditional capacity needs to be reduced. Finally, as of April 2022, Experity reported that non-COVID visit volumes were only at 72% of pre-COVID levels. This trend means we cannot peg a complete return to pre-COVID levels because volumes have not recovered despite life returning to normal in many states. With all these challenges, we can no longer use 2019 as a benchmark since the landscape of urgent care volume mix has changed dramatically over the last two and a half years. As a result, we elect to use the average of the last six to eight months to capture seasonality and current trends. Although these volumes trend lower than 2019, they reflect the changing reality of the volume mix and capacity concerns.
Once we have determined go-forward monthly volumes for the three test types, we can make the appropriate quality of earnings adjustments. We begin by creating cash waterfalls for each volume type to calculate accrual basis revenue. Using this revenue, we calculate the historical average reimbursement for each visit type. Due to changing reimbursement trends for COVID testing and office visits in recent years, we use the average of the last six months in our calculation of adjusted revenue. Once we have determined the revenue adjustment, we estimate the associated variable expense impact for each visit type based on common size percentages, direct allocation, and invoice review. Volume estimates are the key to the calculation of both the revenue and expense adjustments, which emphasizes the importance of reasonable estimates.
Additional COVID-Related Quality of Earnings Adjustments
Besides impacting urgent care volume and mix, COVID-19 also impacted the ramp up of de novo facilities opened just before and during the pandemic. Average startup location volumes historically averaged between 10 to 20 visits per day in the first six months of operations for de novo locations opening between 2016 and 2019. Volumes for these locations stabilized at approximately 30 – 40 patients per day after 18 to 24 months. However, startup locations that opened in 2021 saw an average of 32 patients per day in the first month, and 60 visits per day after just six months.
For a quality of earnings analysis, the changes to the ramp up of de novo facilities create a challenge for quantifying a run-rate adjustment. First, there are concerns about sustainability of patient volume as COVID testing declines. In the beginning of the pandemic, shortages of tests meant individuals sought tests wherever possible even if it meant traveling to a further center. Some parent companies even made de novo locations into COVID-only sites to raise awareness of the new location. As a result, some patients may return to a different location for future visits which creates uncertainty when estimating the impact of a return of traditional urgent care volume. To combat this challenge, we engage in discussions with management to identify existing sites that serve a similar demographic and can be used as the volume being averaged to calculate run-rate.
The case mix changes also cause issues in conducting a revenue hindsight analysis. Due to government mandates around reimbursement for COVID testing, UCCs experienced higher reimbursements for patients getting a COVID test since many payors were obligated to pay all claims. Additionally, many COVID tests were administered either with no office visit or with an office visit of a lower E&M code level than typical. Furthermore, UCCs that did require an office visit often saw new patient visits becoming a greater ratio of total visits following COVID spikes and new patients represented over 50% of patient visit mix after April 2020 and into 2021. This patient mix shift drove an increase in average net revenue per visit for many UCCs. Finally, stay-at-home orders and people working from home have driven a slowdown in reimbursements from payors. As a result, historical collection trends were skewed during 2020 and into 2021. All these factors necessitate a close analysis of the revenue data for UCCs which often entails performing separate analysis by visit type (i.e., test vs. non-test) or CPT code (i.e., new patient vs. established). Due to the changing landscape of patient and visit type mix since the beginning of the pandemic, the revenue hindsight analysis has become a large area of focus for all parties to the transaction.
Typically, the last, most straightforward adjustments we perform in our analysis center on non-recurring COVID revenue and expenses such as CARES Act funding and rent abatement. Many UCCs participated in the various economic relief programs and received Paycheck Protection Program (PPP) loans, HHS stimulus grants, Economic Injury Disaster Loans (EIDL), etc. As these amounts were received or forgiven, many UCCs recognized the amounts as income. Additionally, UCCs often took advantage of temporary rent or payroll tax abatements that were recaptured or will be recaptured at a later date. These amounts represent non-recurring or temporary measures, and we always include adjustments to eliminate their impact from earnings.
Conclusion
With all these complications and uncertainties arising from COVID testing, both buyers and sellers in the UCC market need to engage a quality of earnings team for their transactions. Over the last two and a half years, the outlook of COVID and testing has changed week-to-week and month-to-month. During 2021, our team was engaged as a sell-side advisor and conducted an original analysis in addition to two roll forwards for one client. Each of our analyses was at a different point in 2021 and each analysis had unique trends around vaccination, variants, and seasonality. By engaging a quality of earnings team, our client was able to get a deeper analysis of its earnings to quantify the effects of this changing landscape on its EBITDA. From the expansion of at-home testing to the potentially permanent depression of traditional volume, quality of earnings will be crucial in analyzing the changing nature of the COVID and UCC landscape as we continue to return to normal.
Categories: Uncategorized
Utilizing Telehealth Services to Improve Access to Behavioral Healthcare
August 9, 2022
By: Mallorie Holguin, Preston Edison, and Dane Hansen
Over the past few years, trends and events have occurred that have led to increased and continuing demand for mental health care services. First, the Affordable Care Act (ACA) expanded coverage and access to mental health care services.[1] Then, more recently, the COVID-19 Public Health Emergency (“PHE”), and corresponding citywide shutdowns, brought about a spike in anxiety and depression with these conditions increasing to four times pre-COVID-19 levels.[2,3] Healthcare workers were among some of the most heavily impacted with one study finding that almost half of healthcare workers reported serious psychiatric symptoms, including suicidal ideation.[4] While demand for mental health services has continued to increase, the number of providers actively practice in the United States is estimated to have the capacity to meet only 28% of all mental healthcare needs.[5]
As the COVID-19 pandemic increased demand for mental health care services, the healthcare industry rapidly expanded its offering of telehealth services. Specifically, telehealth services grew to represent up to 40% of outpatient care at the peak of the COVID-19 pandemic (up from less than 1% of outpatient care in 2019).[6] This increase in service offerings and patient care in the telehealth space was made possible by relaxed regulations related to the provision of telehealth services.[7] In the following sections, we discuss how healthcare organizations can implement or continue to expand telehealth services to meet demand for mental health care services in the communities they serve.

Implementing Telehealth / Virtual Care Services to Expand Access
As discussed, the gap between the supply of mental health providers and the demand for mental health services is notably widening. As of June 30, 2022, Health Resources and Services Administration (HRSA) has designated 6,300 mental health provider shortage areas.[8]

These designated shortage areas collectively contain over 152 million Americans, approximately 46% of the total population. As health systems and hospitals attempt to navigate these challenges, telemedicine has emerged as a potential avenue for bridging the gap between the supply and demand for behavioral health services.
The American Telemedicine Association (“ATA”) describes telemedicine as the “natural evolution of healthcare in the digital world.” Precisely, telemedicine promotes and improves the quality, access and affordability of healthcare through the use of rapidly evolving technologies. Specifically, telemedicine refers to the use of medical information exchanged between parties via electronic communications to improve a patient’s clinical health status. Electronic communication including videoconferencing, streaming media, transmission of still images, remote patient monitoring devices and many other telecommunication methods allow(s) physicians to closely monitor and/or provide clinical services that would otherwise be unavailable for the patient. Oftentimes, the electronic information is combined with electronic medical records (“EMR”) to formulate a more accurate consultation or specialist opinion. Telehealth allows practitioners and patients to interact without the requirement to be face-to-face in a hospital or clinic setting.
At the same time, remote, or tele-, work was implemented across many industries to combat the challenges of the COVID-19 PHE shutdowns. As a greater percentage of the workforce had the option to participate in a remote work setting, 9 in 10 remote workers want to maintain remote work to some degree going forward.[9] One of the top reasons employees desire a hybrid or fully remote work arrangement is that it increases personal wellbeing. Given the well-documented physician burnout rates exacerbating provider shortages, it would be prudent for health systems, hospitals, and practitioners to consider using alternative coverage models, including employing the use of telemedicine. By leveraging virtual care offerings, practitioners can experience the same advantages that have led the majority of Americans to respond with resounding positivity to remote work, potentially alleviating some of the stressors that contribute to provider burnout.
Telemedicine offerings can also be used to redistribute the supply of practitioners. The hub and spoke model was one of the first practical telehealth models and is a common way to structure virtual care offerings while leveraging the existing practitioner base and extend care to facilities or communities in need. In this model, the hub facility is typically a larger facility that has the resources to provide specialized care that many smaller and/or rural facilities lack. By scaling the existing resources of the hub, the spoke sites are able to close gaps in care without incurring the costs associated with a full-time provider or locum tenens staffing. Behavioral health providers focused on increased access to care and better quality of care outcomes for their patients will find success in a virtual care-driven future.
Telemedicine is a tool for healthcare entities that, if embraced and properly utilized, can help bridge the behavioral health care gap. To effectively leverage virtual care services, it is important to understand the compliance and regulatory implications of these offerings and to establish equitable compensation models for providers that consider any limitations remote workplaces on of their scope of practice.
Financial & Operational Considerations for Behavioral Health Operators
As of July 15, 2022, the COVID-19 PHE was extended through October 2022 by the Department of Health and Human Services (HHS) and, along with it, continued flexibility around regulatory compliance regarding telehealth and reporting deadlines. VMG’s Coding, Compliance, and Operational Excellence (CCOE) division has compiled current documentation and coding requirements for telehealth services, which are listed below. This list is not intended to be exhaustive, but rather an overview of important considerations related to a compliant telehealth service line.
Documentation Requirements:
- Patient’s Verbal Consent
- Modality (Audio + Video, Audio Only)
- Location of Patient
- Location of Provider
- Session Start and End Time
- Total Session Time
- Add appropriate billing modifiers:
- 93: Synchronous Telemedicine Service Rendered Via Telephone or Other Real-Time Interactive Audio-Only Telecommunications System
- 95: Telehealth Service
- FQ: Behavioral health audio-only services
In addition, the following guidelines should be considered when submitting claims to Medicare for virtual mental health services:
- If the service is performed via audio only, the practitioner must have the capacity for real-time audio and visual technology.
- The face-to-face service may be with a clinician of the same specialty in the same group.
- The patient must be seen in-person once every 12 months, unless it is determined that would be “inadvisable or impractical” for the patient.
- If the patient is not seen in person once every 12 months, the reason for the exception must be documented in the medical record.
- Initial evaluations, psychotherapy, and crisis psychotherapy may be performed via real-time audio and visual communication or audio only.
- Evaluation and Management (E/M) services (office visits 99202–99215) require real-time audio and visual technology.
- Telephone E/M Services (99441-99443) require audio only.
Additionally, in its CY 2023 Proposed Rule, CMS has proposed to make hospital outpatient behavioral telehealth services reimbursement permanent, which could increase access to behavioral health services in rural and other underserved communities.[11] It is important to note that after the PHE ends, additional behavioral health and telemedicine requirements will need to be met including:
- The patient must have had a face-to-face service with the clinician within 6 months of starting telehealth (except for substance use disorders treatment and patients in a geographically underserved area).
- The face-to-face service may be with a clinician of the same specialty in the same group.
- The patient must be seen in person once every 12 months, unless it is determined that would be “inadvisable or impractical” for the beneficiary.
- If the patient is not seen in person once every 12 months, the reason for the exception must be documented in the medical record.
As virtual services become more common through further regulatory shifts, healthcare organizations can expect increased scrutiny towards telehealth services arrangement by governmental enforcement bodies. The Office of Inspector General (OIG) and Department of Health and Human Services (HHS) released a Special Fraud Alert (Alert) on July 20, 2022, related to the inherent fraud and abuse risk associated with physicians or other health care professionals entering into arrangements with telemedicine companies, which specifically addresses fraud schemes related to telehealth, telemedicine, or telemarketing services based on dozens of civil and criminal investigations. The Alert identified seven characteristics that the OIG believes could suggest a given arrangement has potential risk for fraud and abuse. To learn more, reference this article and OIG’s statement.
Conclusion
By using telehealth, behavioral health providers can better fill the gap between growing demand and limited supply, providing quality and efficient services to those in need, particularly to underserved and isolated communities. Compliant telehealth arrangements can promote more efficient financial operations for health systems, provide increased access to care for patients, and improve the well-being of behavioral health providers.
Sources:
1. https://www.commonwealthfund.org/blog/2020/aca-10-how-has-it-impacted-mental-health-care
2. https://www.psychiatrictimes.com/view/psychiatric-care-in-the-us-are-we-facing-a-crisis
4. https://pubmed.ncbi.nlm.nih.gov/33267652/
8. https://data.hrsa.gov/topics/health-workforce/shortage-areas
9. https://news.gallup.com/poll/355907/remote-work-persisting-trending-permanent.aspx
Categories: Uncategorized
What the FMV? Do You Have a Commercial Reasonableness Problem?
August 4, 2022
By: Bartt B. Warner, CVA and Mallorie Holguin
The following article was published by the American Association of Provider Compensation Professionals.
Hospitals and health systems have historically focused on fair market value (FMV) when there is a financial relationship with a physician. The reason for this is that the healthcare landscape is highly regulated and includes the Stark Law, the Antikickback Statute, the False Claims Act (FCA), and Internal Revenue Service 501(c)(3) status for tax-exempt entities whereas violations could result in exorbitant penalties, sanctions or even jail time. Meanwhile, these health systems are still reeling from the impact of COVID-19 and the recent changes to the Medicare Physician Fee Schedule (MPFS). In light of this, less focus has been placed on both defining and assessing commercial reasonableness.
As a result, hospitals and health systems may have commercial reasonableness issues that will further be exacerbated unless a fundamental shift happens and as much focus is placed on commercial reasonableness as is currently done with ensuring compensation arrangements are consistent with FMV. Part of the challenge stems from the fact that the process of determining commercial reasonableness is not as well defined and requires a thorough understanding of the specific facts, circumstances, and rationale of the arrangement. While the healthcare valuation community has refined the FMV process and determination of value over the past decade, the concepts, and methodologies for determining commercial reasonableness on its own terms have not yet been succinctly defined or standardized. However, increased government scrutiny underscores the need to understand and ensure that this requirement is met for all financial arrangements between referring parties.
Defining Commercial Reasonableness
As previously mentioned, the definition for commercial reasonableness has historically not been explicitly defined; however, on December 2, 2020 the new Stark Law Final Rule1 provided a clearer picture with the following definition, “commercially reasonable” means “the particular arrangement furthers a legitimate business purpose of the parties to the arrangement and is sensible, considering the characteristics of the parties, including size, type, scope and specialty.” [2] Prior to the new Stark Law Final Rule, commercial reasonableness was commonly interpreted by relying on guidance from the 1998 proposed rule which stated, an arrangement is commercially reasonable if it “appears to be a sensible, prudent business agreement, from the perspective of the particular parties involved, even in the absence of any potential referrals.” [3] In addition, the new Stark Law Final Rule clarified the issue of profitability. Specifically, that an arrangement may be considered commercially reasonable, even if it does not result in profit for one or more of the subject entities.
“The determination that an arrangement is commercially reasonable does not turn on whether the arrangement is profitable; compensation arrangements that do not result in profit for one or more of the parties may nonetheless be commercially reasonable…[4] We acknowledge that, even knowing in advance that an arrangement may result in losses to one or more parties, it may be reasonable, if not necessary, to nevertheless enter into the arrangement.” [5]
However, one should note that commentary from prior court decisions have looked unfavorably regarding commercial reasonableness and unprofitable arrangements where physician compensation exceeded professional collections. It is yet to be determined how the courts will interpret this new guidance, but the Centers for Medicare & Medicaid Services (CMS) provided further guidance with the following statement:
“Although we believe that compensation arrangements that do not result in profit for one or more of the parties may nonetheless be commercially reasonable, we are not convinced that the profitability of an arrangement is completely irrelevant or always unrelated to a determination of its commercial reasonableness, for instance, in a case where the parties enter into an arrangement aware of its certain unprofitability and there exists no identifiable need or justification- other than to capture the physician’s referrals- for the arrangement.” [6]
This commentary provided much needed guidance for health systems as they navigate through the complexities of physician compensation and profitably. As part of the commentary on profitability, CMS noted a non-exhaustive list of reasons why parties might appropriately enter into arrangements that result in losses, including: [7]
- Timely access to health care services;
- Community need;
- Fulfillment of licensure or regulatory obligations;
- The provision of charity care; and,
- The improvement of quality and health outcomes.
Although, CMS has provided guidance on commercial reasonableness, it is important to note that this guidance is still limited. Therefore, it is imperative that hospitals and health systems mitigate their risk by having a systematic and standard approach to commercial reasonableness and seek the expertise and assessments from third-party valuation firms that are focused on healthcare. The process should begin with the following:
- Inventory all physician compensation arrangements;
- Determine internal thresholds of when an arrangement needs to be sent to a third-party valuation firm for a commercial reasonableness assessment;
- Ensure the compliance plan is consistent;
- Perform a risk assessment on the physician compensation arrangements; and,
- Thoroughly document and support internally or send to an independent third-party valuation firm for a commercial reasonableness assessment.
When assessing the commercial reasonableness of an arrangement, a broad lens must be taken, and an analysis of both qualitative and quantitative factors must be considered. As such, when evaluating the commercial reasonableness of an arrangement, one must first start with if the arrangement represents a sensible and prudent business decision without the consideration of referrals. In other words, is there a legitimate reason as to why the parties would enter into this arrangement, excluding the potential for referrals.
Generally, VMG Health recommends documenting the commercial reasonableness of an arrangement with consideration to each of the following factors.
Fair Market Value Standards
If the compensation terms of the arrangement fail to meet FMV standards, as defined by CMS, the arrangement will also fail to meet commercial reasonableness standards. The FMV assessment should consider the specific services being provided, specialization and expertise of the provider, as well as whether the compensation paid by the parties would be the same as that resulting bona fide bargaining between well-informed parties that are not in a position to generate business for one another. A separate third-party valuation firm may be contracted to opine on the FMV of the compensation depending on the risk tolerance of the hospital or health system.
Economic & Financial Considerations
If the compensation terms of the arrangement have been determined to be within FMV, prudent practice suggests documenting both qualitative and quantitative factors driving the compensation terms. These specific factors may include some or all of the following: 1) business purpose of the arrangement, 2) the arrangement’s impact on the strategic and financial goals of the contracting entity, 3) a financial analysis of the arrangement and the impact on the contracting entity, and 4) contributing national, regional, and local economic conditions. Generally speaking, economic conditions and the supply and demand for physician services will influence the financial impact to the health system. For example, a subspecialized physician with a unique skillset may fill a gap in care and allow patients to receive care within their community (business purpose and quality of care goals) but will likely demand premium compensation (low supply of subspecialists) and may not see as many patients as those physicians treating more general medical issues (low percentage of population with unique medical condition(s).
As noted previously, CMS has indicated that the commercial reasonableness of an arrangement does not hinge on whether one or more of the parties earn a profit. That said, should an arrangement result in a net loss to a hospital or health system, it is prudent to document the factors contributing to the loss (e.g., a physician spending 50% of their time in a non-revenue-generating directorship role) and how the loss compares to other similar arrangements with other similar providers.
Operational Considerations
Outside of the direct strategic and financial goals of the parties, the arrangement may also benefit the contracting entity in a non-financial manner; some of which may overlap with the economic considerations discussed above. These factors may include how the arrangement or subject provider supports other service lines within the organization, expected improvements in patient access or quality of care, and/or other service line development considerations.
Service Provider Considerations
Finally, a robust commercial reasonableness assessment should include documentation and commentary related to the specific provider’s ability to meet the goals and provide the services outlined in the previous two sections. This type of documentation may include a summary of the provider’s training and experience, operational reports for the provider demonstrating their capacity to hit projected production targets, quality scorecards, etc. Of particular importance in stacking arrangements is confirmation and attestation by management that the specific provider can perform all of the services outlined in the arrangement without compromising patient safety or quality of care. As a conservative measure, some organizations may choose to include production targets or performance measures to ensure all of the service components of the stacking arrangement are satisfied.
Conclusion
Although there has been a historical focus on assessing if an arrangement is consistent with FMV, recent enforcement and shifts in health system risk tolerance should result in a greater emphasis in both assessing and determining the commercial reasonableness of arrangement. While the vast majority of documentation and information required for a commercial reasonableness assessment will come from within an organization, an outside FMV analysis and commercial reasonableness assessment will provide independent support and assurance that the factors above have been considered and reduce an organizations’ overall compliance risk.
Endnotes:
- 85 Fed. Reg. at 77492 (Dec. 2, 2020).
- 85 Fed. Reg. at 77657, codified at 42 C.F.R. § 411.
- 63 Fed. Reg. at 1700.
- 85 Fed. Reg. at 77431.
- 85 Fed. Reg. at 77531.
- 85 Fed. Reg. at 77531.
- 85 Fed. Reg. at 77534.
Categories: Uncategorized
Private Equity: Piqued Interest in Medical Physics
August 2, 2022
By: Maxwell Swan, Savanna Dinkel, CFA, & Vincent M. Kickirillo, CFA
The private equity (PE) space is breaking records as the world continues to emerge from the COVID-19
pandemic. PE fundraising surged almost 20 percent in 2021 as firms looked to jump back in after the
uncertain financial climate created by the pandemic. When looking to deploy this capital, PE firms have
continued to take an interest in the healthcare industry. (1) Recently within this industry, PE firms made
investments in the $4.47 billion medical physics industry that has maintained a 5.9 percent CAGR from
2013 through 2021. There are numerous reasons why PE firms have increasingly targeted the medical
physics industry, such as the current industry composition along with the growth in the need and use of
the specialty. (2) These characteristics set medical physics apart as a particularly interesting area for future
investment.
Medical Physics Industry Landscape
Medical physics is a healthcare specialization focused on the application of physics to the treatment and
diagnosis of disease. Most often, medical physics is seen in the form of nuclear medicine, diagnostic
imaging, and radiation oncology. The medical physics industry is made up of numerous small-scale
providers that operate in localized geographical areas. Only a handful of substantially sized enterprises
operate in the medical physics space, resulting in a highly fragmented industry ripe for acquisitions and
roll-ups into large-scale platforms. The fragmentation of the industry provides ample opportunities for PE
to enter and expand its foothold in the medical physics industry. (3)
In addition to the extreme fragmentation, the demand for medical physics is expected to grow
significantly over the next six years. Experts predict the medical physics market will grow at a healthy 6.2
percent CAGR through 2028, exceeding a $6 billion market valuation. This growth is driven by the
increasing adoption and widening horizons of nuclear medicine across the healthcare landscape. (2)
Additional growth is expected as hospital consolidation continues to increase the use of outsourced
medical services. Even medical tourism is expected to contribute to industry growth as revenue comes in
from those traveling to seek specialized medical care from countries like China, Brazil, or India. (4) This
multisource growth is an appealing attribute for PE capital looking for favorable returns.
Lastly, significant barriers to entry exist for new medical physics operations, including high capital
requirements for expensive machinery, increasing regulation required for the specialty, and most
notably, the shortage of skilled providers in the medical physics space. In 2014 a mandatory residency
was implemented to better prepare new medical physicists for the complex field. While the new program
has produced well-prepared providers, it has also created a bottleneck that has put a strain on the
industry’s ability to create new operations. (5) This shortage places established operators with experience
at a significant advantage, setting them up as a prime target for PE investment.
Private Equity Investment Considerations
PE firms can be beneficial collaborators and partners to medical physics practices. As PE interest in the
healthcare industry continues to increase, modern PE firms have gained the expertise to be effective
partners to healthcare practices. One of the most effective ways PE firms can enhance a medical physics
practice is through economies of scale. PE firms allow businesses to take advantage of efficiencies
created through economies of scale. By improving and centralizing back-office business operations and
providing greater access to technology, medical data, reporting and tracking systems, consolidated purchasing power, and marketing, private equity partners can create a more efficient business
structure and free up providers to focus on patient care.
Similarly, continued hospital consolidation may require other providers within their spheres of
influence to meet the greater demands and specialization needed in the industry. Some of
these demands include the growing regulation required of medical physics practices. (6)
Increasing regulatory demands may put monetary and staffing pressure on smaller
operations. The resources offered by PE investment could help alleviate some of these
pressures. (7) Furthermore, these resources could potentially improve the negotiating power
of businesses, resulting in better commercial payor rates and increased earnings.
Finally, PE investors could provide exit opportunities for retirement-age providers. PE
investment offers an exit strategy that enables these providers to monetize the business they
have built while also allowing the business to remain as an employer and provider of needed
care in its respective community. Based on an examination of the industry, as well as
discussions with industry professionals, sellers of a medical physics practice may be able to
expect a middle single-digit multiple on a given transaction. (4) For platform transactions, high
single-digit or low double-digit multiples may be warranted in the market.
Major Players and Recent Activity
As PE groups increase their interest in the medical physics industry, there have already been
several notable deals. Below is a summary of a few recent acquisitions, partnerships, and
platforms:

Blue Sea Capital, a PE firm based in Florida with over $750 million in assets, partnered with
mid-Atlantic firm Krueger-Gilbert Health Physics, LLC in April 2019 to form the platform
company Apex Physics Partners. Soon after, Apex entered partnerships with Ohio Medical
Physics Consulting, National Physics Consultants, Radiological Physics, and ZapIT! QA to
enter the Ohio, Texas, and New Mexico markets. (8) In 2021 Apex added several new
partnerships including Texas-based D. Harris Consulting, Indiana-based Advance Medical
Physics, Indiana-based INphysics, and Pacific Island-based Gamma Corporation to its
partnerships as the firm continued its expansion into new markets. (10, 11, 12, 13, 14)

L2 Capital, a PE firm based in Pennsylvania with over $100 million under management,
acquired Associates in Medical Physics, LLC and Radiation Management Associates, LLC in
May of 2017. L2 combined the medical physics service companies to create the platform
company Aspekt Solutions in April of 2021. In May of 2021, Aspekt Solutions acquired Nordic
Medical Physics to expand its geographical reach. (15, 16, 17)

LNC Partners, a PE firm with $500 million under management, completed a recapitalization
of West Physics Consulting, LLC in May 2018. West Physics has since acquired Phoenix
Technology Corporation and Radiological Physics Consultants, Inc. to become the largest
diagnostic medical physics practice in the US. (18) West Physics operates in all 50 US states,
its federal territories, the Caribbean, and the Middle East. (19, 20)

Fortive Corporation is a publicly traded, diversified industrial technology conglomerate
company. Landauer provides outsourced medical physics services worldwide. Previously
involved with Gilead Capital and T. Rowe Price Associates, Landauer was acquired by Fortive
Corporation in October 2017. (21)
The Future of Investment in the Medical Physics Industry
The medical physics industry is increasingly becoming a hot target of PE investment. Although a
few major players are emerging and consolidation is increasing, there are plenty of
opportunities for PE partnerships to gain size and industry leverage due to the sheer number of
small operators in the medical physics space. The benefits and resources brought by PE firms
may be increasingly enticing to medical physics operators as the healthcare industry evolves. (6)
The spread of usage, science, treatment, and understanding of the industry will continue to
increase the demand for the care that these medical physics specialists provide.
Sources:
1. “McKinsey’s Private Markets Annual Review.” McKinsey & Company. March 24, 2022.
2. “Medical Physics Market Report.” Future Market Insights. March 2022.
3. “Increased M&A in Medical Physics—What It Means to Business Owners.” SC&H Group. June 13, 2018.
5. “Where Have All the Medical Physicists Gone?” Aspekt Solutions. April 12, 2022.
6. “Medical Physics M&A: Industry Consolidation Outlook.” SC&H Group. July 2, 2019.
7. “Increased M&A in Medical Physics–What It Means to Business Owners.” SC&H Group. June 13, 2018.
8. “Apex Physics Partners Announces Growth Investment.” Blue Sea Capital. May 7, 2019.
13. “Indiana’s Leading Therapy Medical Physics Group, INphysics, joins Apex Physician Partners.” Apex Physics. September 15, 2021. Press Release.
15. “L2 Capital Announces Healthcare Services Platform Acquisition.” L2 Capital. May 8, 2017.
17. “Aspekt Solutions Acquires Nordic Medical Physics.” Business Wire. May 24, 2021.
18. “LNC Partners Completes Recapitalization with West Physics Consulting.” LNC Partners. May 29, 2018.
20. “West Physics Acquires Phoenix Technology Corporation.” LNC Partners. May 13, 2019.
21. “Landauer, Inc. Private Company Profile.” Capital IQ. May 23, 2022.
Categories: Uncategorized
Reflecting on Recent Regulatory Changes to Stark Law: A Real Estate and Equipment Valuation Perspective, Part 1 & 2
August 1, 2022
By Frank Fehribach, MAI, MRICS, Nick Shannon, ASA, Joel Gomez, ASA, and Grace McWatters
VMG Health was published in The Complete Guide to Fair Market Value Under the Stark Regulations. This publication was authored by Timothy Smith and is a collaboration between Business Valuation Resources, LLC and the American Association of Provider Compensation Professionals (AAPCP).
The sections, “Reflecting on Recent Regulatory Changes to the Stark Law: A Real Estate and Equipment Valuation Perspective, Part 1 & 2” were written by VMG Health experts Frank Fehribach, Nick Shannon, Joel Gomez, and Grace McWatters.
In their sections, the authors offer an in-depth overview of fair market value (FMV) and provide insight into the impact of the updates to the Stark Law from a real estate and capital asset valuation perspective.
About the Publication
This up-to-date guide provides a comprehensive and essential evaluation of the revised definitions of fair market value (FMV) within the latest amendments to the federal physician self-referral law, commonly known as the Stark law. The recent regulatory updates introduce significant alterations to recognized valuation principles and methodologies for determining FMV under the Stark Law.
For additional guidance related to real estate valuation, capital assets valuation, or any other services, our experts would be happy to assist. Please email info@vmghealth.com to learn more.
Categories: Uncategorized
OIG Puts the Spotlight on Practitioner Arrangements with Telemedicine Companies in a Special Fraud Alert
July 21, 2022
By: Bartt Warner, CVA and Dane Hansen
The Office of Inspector General (OIG) and Department of Health and Human Services (HHS) released a Special Fraud Alert (Alert) on July 20, 2022 related to the inherent fraud and abuse risk associated with physicians or other health care professionals entering into arrangements with telemedicine companies (Telemedicine Companies).1 Specifically, addressing fraud schemes related to telehealth, telemedicine, or telemarketing services based on dozens of civil and criminal investigations. The Alert identified seven characteristics that the OIG believes could suggest a given arrangement has potential risk for fraud and abuse. However, the OIG was cautious not to state that all Telemedicine Companies and arrangements are suspect, but rather to identify key characteristics in potentially problematic arrangements as the prevalence of telehealth services continually increases. In addition, the Alert was designed to provide practical compliance guidance and help establish guardrails with relation to telemedicine arrangements. Simultaneously, the OIG also updated its Telehealth Resource Page2 which aggregates compliance and enforcement resources.
Kickbacks
The Alert provided a specific example of how Telemedicine Companies have utilized kickbacks to aggressively recruit and reward telemedicine practitioners to further their fraud schemes. According to the example:
“…in some of these fraud schemes Telemedicine Companies intentionally paid physicians and nonphysician practitioners (collectively, Practitioners) kickbacks to generate orders or prescriptions for medically unnecessary durable medical equipment, genetic testing, wound care items, or prescription medications, resulting in submissions of fraudulent claims to Medicare, Medicaid, and other Federal health care programs. These fraud schemes vary in design and operation, and they have involved a wide range of different individuals and types of entities, including international and domestic telemarketing call centers, staffing companies, Practitioners, marketers, brokers, and others.”
Based on the OIG’s experience with fraud and abuse in this realm, the Telemedicine Companies often work out an arrangement with the Practitioners to order and prescribe medically unnecessary items and services. Oftentimes, the Telemedicine Companies pay the Practitioners for prescribing items or various services to patients who have had limited interaction with the Practitioner and without regard to the medically necessity for this service or prescription. In addition, these kickbacks are routinely disguised as payment per review, audit, consult, or for the assessment of medical charts and are often tied to the volume of federally reimbursable items or services ordered or prescribed by the Practitioners. As a result, the fees associated with the problematic arrangements are being used as a mechanism to incentivize a Practitioner to order medically unnecessary items or services according to the Alert. Of additional concern, the OIG noted that in many cases the Telemedicine Companies sell the prescriptions that are generated by the Practitioners to other entities who in turn will fraudulently bill for the medically unnecessary items or services.
Compliance Risk
The OIG makes it clear in the Alert that these fraudulent telemedicine schemes pose a significant risk to the health care system and that both Practitioners and health systems should exercise extreme caution when entering into arrangements with Telemedicine Companies. Specifically, the Alert stated,
“These schemes raise fraud concerns because of the potential for considerable harm to Federal health care programs and their beneficiaries, which may include: (1) an inappropriate increase in costs to Federal health care programs for medically unnecessary items and services and, in some instances, items and services a beneficiary never receives; (2) potential to harm beneficiaries by, for example, providing medically unnecessary care, items that could harm a patient, or improperly delaying needed care; and (3) corruption of medical decision-making.”
These telemedicine schemes have the potential for violating multiple Federal Laws, but most specifically, the Federal anti-kickback statute. Violations of the Federal Anti-kickback statute ascribes liability to both sides of the arrangement and can potentially lead to criminal, civil, or administrative liability under other Federal laws as well.
Suspect Characteristics
Based on the OIG’s experience with various problematic telemedicine arrangements, seven “suspect characteristics” were identified that taken together, or separately, could suggest an arrangement presents a heightened risk for fraud and abuse. However, it should but noted that the list is not exhaustive, but rather illustrative and the presence or absence of these characteristics does not determine if a particular telemedicine arrangement would constitute grounds for legal sanctions.
- “The purported patients for whom the Practitioner orders or prescribes items or services were identified or recruited by the Telemedicine Company, telemarketing company, sales agent, recruiter, call center, health fair, and/or through internet, television, or social media advertising for free or low out-of-pocket cost items or services.
- The Practitioner does not have sufficient contact with or information from the purported patient to meaningfully assess the medical necessity of the items or services ordered or prescribed.
- The Telemedicine Company compensates the Practitioner based on the volume of items or services ordered or prescribed, which may be characterized to the Practitioner as compensation based on the number of purported medical records that the Practitioner reviewed.
- The Telemedicine Company only furnishes items and services to Federal health care program beneficiaries and does not accept insurance from any other payor.
- The Telemedicine Company claims to only furnish items and services to individuals who are not Federal health care program beneficiaries but may in fact bill Federal health care programs.
- The Telemedicine Company only furnishes one product or a single class of products (e.g., durable medical equipment, genetic testing, diabetic supplies, or various prescription creams), potentially restricting a Practitioner’s treating options to a predetermined course of treatment.
- The Telemedicine Company does not expect Practitioners (or another Practitioner) to follow up with purported patients nor does it provide Practitioners with the information required to follow up with purported patients (e.g., the Telemedicine Company does not require Practitioners to discuss genetic testing results with each purported patient).”
Best Practices
Given the heightened regulatory scrutiny placed on telehealth service arrangements by the OIG and HHS, it is pertinent for health systems, hospitals, and Practitioners to employ certain best practices when considering, implementing, and operating any type of virtual care program. Many of the best practices for traditional face-to-face professional services arrangements are directly applicable to telemedicine arrangements. For example, a telehealth arrangement should be justifiable for all parties involved. From the perspective of a health system or hospital for example, the addition of a virtual care service line could be pursued to fill a highly desired gap in medical care, increase the quality of medical care currently available to its patient base, or alleviate overburdened Practitioners.
Prior to entering any business relationship for telehealth services, it is a best practice to consult with legal counsel. Telemedicine service contracts should be explicit in outlining the expectations of medical care and the structure and magnitude of remuneration. Further, it is particularly important to maintain ongoing dialogue with legal counsel throughout the life of a telemedicine relationship to ensure that a program that was once compliant does not break the bounds into non-compliance over time. Preserving a compliant telehealth business relationship is typically aided by a robust compliance program, created with the help of legal counsel, which outlines specific guidelines for professional examinations, prescribing and billing practices, administrative and record maintenance procedures. Compliance programs should be consistently reviewed and updated as regulatory bodies issue more literature on the subject matter. A static compliance program may quickly become inadequate as the virtual care regulatory landscape continues to evolve. Obtaining third party support of an arrangement is often a pillar of successful compliance programs. The arrangement should be commercially reasonable to all parties involved and any compensation should be directly attributed to services performed and have no consideration of referrals. Obtaining a third-party fair market value (FMV) review is a key best practice in maintaining regulatory compliance within the context of telemedicine arrangements. In addition, seeking a third-party commercial reasonableness assessment should also help mitigate compliance risk by documenting and assessing both qualitative and quantitative factors as to why the telemedicine arrangement is a sensible and prudent business decision without the consideration of referrals.
Conclusion
Although the Special Fraud Alert addresses that it is not intended to discourage legitimate telemedicine arrangements, serious compliance risk may occur if an arrangement has any of the seven “suspect characteristics” as previously discussed. In addition, the OIG made it clear that Practitioners have both legitimately and appropriately utilized telehealth services to provide medically necessary care to their patients during the current public health emergency. Best practices should include a cautious approach to telemedicine arrangements while ensuring there are specific guidelines and guardrails, determining and documenting the justification for the arrangement, ensuring the arrangement is commercially reasonable, and determining if the remuneration paid to the Practitioners is consistent with FMV.
Endnotes
1 Special Fraud Alert: OIG Alerts Practitioners To Exercise Caution When Entering Into Arrangements With Purported Telemedicine Companies available at: https://oig.hhs.gov/documents/root/1045/sfa-telefraud.pdf
2Telehealth Featured Topic from HHS and the OIG available at: https://oig.hhs.gov/reports-and-publications/featured-topics/telehealth/
Categories: Uncategorized
Is There Value in Your Inpatient Rehabilitation Facility?
June 27, 2022
By: Sydney Richards, CVA and Stephan Peron, CVA
Inpatient rehabilitation utilization has experienced remarkable growth over the past decade, fueled by a demographic boom in the industry’s primary patient population, stable regulations, and continued Medicare reimbursement increases. Per the 2022 MedPac Report [1], inpatient rehabilitation facilities (IRFs) accounted for $8 billion of Medicare spending in 2020, and paid out to about 1,160 IRFs and inpatient rehabilitation units (IRUs) nationwide. IRUs are operated as distinct part units of an acute hospital compared to an IRF which is a licensed freestanding facility.
As the Medicare population continues to grow [2], and industry data increasingly demonstrates both the clinical efficiency and cost-effectiveness [3] of inpatient rehabilitation compared to alternative post-acute alternatives, inpatient rehabilitation utilization is expected to continue to climb. Additionally, with the low cost of capital and proliferation of strategic post-acute buyers available, it is a seller’s market for IRFs. These trends are causing many acute care operators to question whether they should monetize their hospital based IRU by selling or partnering with a third party.
Selling or partnering an IRU can provide numerous benefits to an acute care provider, including the following:
- An infusion of cash on their balance sheet for selling a portion of or all of a non-core service line
- Turning facility and support services into revenue from the IRF joint venture
- Gaining IRF management expertise to improve acute care discharges and assist with acute length-of-stay
- Freeing up acute care beds
- Improving strategic focus for operational excellence
- Freeing up hospital management time while providing host hospital cash flow
- Protecting from future inpatient rehabilitation regulatory burdens and risks
Benefits of a Joint Venture/Sale of an IRU
The healthcare provider market saw a decrease in the number of transactions in 2020 due to the global pandemic, but according to Irving Levin’s 4th Quarter 2020 M&A report [4] Q4 2020 saw a dramatic recovery in both healthcare deal volume and spending. [3] Based on VMG Health’s 20-year IRF-specific transaction database, IRF average values per occupied bed are trending at a 10-year high. Favorable returns for sellers and buyers are fueling this growth. Post-acute management companies such as Encompass Corp., Kindred Healthcare, and Select Medical have made public their IRF-focused growth objectives and their demand for strategic partnerships with IRFs in markets across the US.
In their September 2020 presentation at the Baird 2020 Global Healthcare Conference, Encompass CEO Mark Tarr reiterated Encompass’s aim to add 100 to 150 beds per year starting in 2021 through de novos and acquisitions. [5] Kindred also stated in a December 1, 2020 press release that they expect “to open 20 new rehabilitation hospitals, acute rehabilitation units, and behavioral health hospitals with leading strategic hospital partners over the next two years.” [6] These factors suggest an opportunity for acute care providers to capitalize on their rehabilitation units through a sale or joint venture model.
Through a sale or partnership, the acute care provider can drive operational excellence for the host hospital by generating additional bed capacity within the health system and potentially lowering the hospital’s average length of stay. An IRF management company with access to best practices in patient admission criteria and IRF regulatory rules could allow for quick patient assessment and discharge from an acute setting to an inpatient rehabilitation facility. If bed capacity or length of stay are not issues for the host hospital, a partnership with a management company could provide additional revenue from facility lease arrangements and patient service contracts. Also, if market demand is present for additional IRF beds, the IRF partnership could use additional nonoperational beds from the host hospital to provide income from otherwise nonprofitable hospital space. Finally, the partnership could license the host hospital’s tradename as another opportunity to capitalize on an existing asset for revenue or joint venture equity.
In addition to driving operational excellence for the host hospital, a sale or partnership with a strategic buyer can advance the IRF’s top-notch quality care and clinical effectiveness at efficient costs. Using knowledge of clinical best practices, therapy recruiting and training, and economies of scale, strategic post-acute providers can drive high-quality care at a more profitable rate and obtain additional returns for the hospital partner. MedPac data corroborates this claim with for-profit aggregate IRF Medicare margins at 24.2% of revenue as compared to nonprofit margins at just 1.5% (see table below).

Additionally, a sale or partnership can insulate an acute care provider from future regulatory burdens or risks. Historically, the inpatient rehabilitation industry has dealt with material legislative changes to patient admissions and quality reporting standards. Specialized post-acute care providers can navigate industry requirements like the Centers for Medicare & Medicaid Service (“CMS”) 60% Rule [7] and the three-hour therapy care rule [8] while providing additional patient access and quality patient care.
As new developments arise, such as continual revisions to the CMS Inpatient Rehabilitation Quality Reporting Program and patient admission criteria, inpatient rehabilitation-specific operators have the concentration and corporate strategy teams in place to study new legislation and its potential impact on operations. Additionally, a sale or partnership can provide a health system with some protection against future reimbursement risks in the industry. MedPac’s annual report to Congress has suggested a 5.0% reduction in the Medicare reimbursement rates for inpatient rehabilitation reimbursement every year from 2017 to the latest report published in March 2022.1 This was in response to the climbing aggregate Medicare margins in the industry, which in 2020 were 13.5% across all IRFs (freestanding and hospital-based, excluding Medicare COVID relief funds), according to the 2022 MedPac report. [1] MedPac estimates the 2020 aggregate Medicare margin was 14.9% including estimated Medicare share of federal relief funds. Although CMS has continued to project positive reimbursement growth in the final rule, if margins continue to rise it is expected that reimbursement may eventually be reduced. Based on the 2020 -0.7% aggregate Medicare margin for nonprofits (2.6% inclusive of federal relief funds) and the 1.6% aggregate Medicare margin for hospital-based IRFs (4.0% inclusive of federal relief funds), it is unlikely that a 5% Medicare reimbursement cut would be viable for many hospital-based IRFs in these settings.
Finally, the sale or partnership of a rehabilitation unit allows the hospital to continue its integrated delivery model with a partner in post-acute care or have a partner ready to implement an integrated network. As CMS transitions to value-based payments and outcome focus, aligning with or selling to a strategic partner could provide the hospital with focused expertise in managing patient readmission. Multiple management companies, such as Encompass, Kindred, and Select, have proven success with risk-sharing models with acute care providers. This could help the health system with both care quality and profitability.
Downsides to a Joint Venture/Sale of IRU
Although there can be many benefits to selling or partnering an IRU, as with any business transaction, there are also some drawbacks. For an acute care provider, a key downside can be the loss of control. The acute care provider would lose the ability to flex its bed use if the inpatient rehabilitation beds were sold or contributed to a joint venture. The ability to flex beds became an important capability during the COVID-19 outbreak in the US. Additionally, under a joint venture partnership, the acute care provider opens itself up to reputation risk since control and management of the IRU is entrusted to a third party. Through a sale or partnership, the acute care provider sells forward profits, and the host hospital needs to account for the overhead expenses that were historically allocated to the IRU.
Conclusion
In summary, many acute care providers should ask, “Are they generating the most value from their IRU as they could or should?” A sale or partnership of the IRF could add value to the health system beyond the initial cash flow from a transaction. The relationship between an acute care host hospital and the IRF is considered a referral relationship with Medicare patients. Therefore, whether through a sale or contribution, healthcare transactions must be properly established at fair market value. Based on VMG Health’s experience with hundreds of IRF transactions, the departmental operational reports never represent IRF’s real economic impact on the host hospital. As many IRUs operate as departments of hospitals and utilize allocated cost methodologies, it can be a highly technical undertaking to assess the profitability and overall value of an IRU. Specific knowledge and insight into key industry value drivers, such as reimbursement/payor mix, staffing metrics, unit size/bed configuration, and utilization, should be considered. A credible valuation with access to a detailed national IRF benchmark analysis9 for revenue and expenses can help ensure a successful transaction.
To provide some IRF value perspective, VMG Health has observed IRF transactions per occupied bed as high as $800,000, with common value ranges per licensed bed between $200,000 and $400,000, and revenue multiples typically falling in the 0.8–1.4x range. This year might be a great time to ask, “Is your IRU generating the value your patients have come to expect, supporting your healthcare strategy, and adding value to your bottom line?”
Footnotes:
[1] MedPac 2022 Report (https://www.medpac.gov/wp-content/uploads/2022/03/Mar22_MedPAC_ReportToCongress_Ch9_SEC.pdf
[2] Medicare population projected at 5% annually over the next several years.
[3] Census projections for 2018 to 2022 and 2022 to 2026. https://www.census.gov/data/datasets/2017/demo/popproj/2017-popproj.html
[4] Encompass Health Investor Reference Book https://s22.q4cdn.com/748396774/files/doc_downloads/investor_reference/2020/11/EHC-Q3-2020-Investor-Reference-Book_11-13-20_As-Filed.pdf
[5] Irvin Levin’s 4th Quarter 2020 M&A Report
[6] Baird 2020 Global Healthcare Conference https://investor.encompasshealth.com/events-and-presentations/other-events-and-presentations/event-details/2020/Baird-2020-Global-Healthcare-Conference/default.aspx
[7] Kindred Healthcare December 1, 2020 Press Release (https://www.kindredhealthcare.com/about-us/news/2020/12/01/kindred-healthcare-advances-growth-strategy-completes-sale-of-rehabcare
[8] Medicare reimbursement per episode of care for inpatient rehabilitation is generally higher as compared to other post-acute facilities because IRFs are designed to offer intensive rehabilitation services to patients that cannot be served in other less intensive rehabilitation settings, such as skilled nursing facilities or home health. Due to the higher reimbursement, CMS became concerned that patients who did not require intensive rehabilitation services were being treated in an IRF setting. Therefore, CMS implemented the 75% rule which required that 75% of patients admitted to an IRF have a primary diagnosis or comorbidity in one of the 13 qualifying medical conditions listed in the table below. After implementing, Medicare adjusted the 75% rule to a new compliance threshold of 60% by the Medicare, Medicaid, and SCHIP Extension Act of 2007 (“MMSEA”). In addition, MMSEA also permitted IRFs to use patient’s secondary medical conditions & comorbidities to determine whether a patient qualifies for inpatient rehabilitative care. The legislation is referred to as the “60% Rule.”
[9] In addition to the 60% rule, to be eligible for payment as an IRF under CMS, patients must attend three hours of therapy in 5 of 7 consecutive days.
[10] VMG Health’s proprietary inpatient rehabilitation database includes 20 years of inpatient rehabilitation analyses with over 100 IRF transactions.
Categories: Uncategorized

