- About Us
- Our Clients
- Services
- Insights
- Healthcare Sectors
- Ambulatory Surgery Centers
- Behavioral Health
- Dialysis
- Hospital-Based Medicine
- Hospitals
- Imaging & Radiology
- Laboratories
- Medical Device & Life Sciences
- Medical Transport
- Oncology
- Pharmacy
- Physician Practices
- Post-Acute Care
- Risk-Bearing Organizations & Health Plans
- Telehealth & Healthcare IT
- Urgent Care & Free Standing EDs
- Careers
- Contact Us
Coronavirus Preparedness and Response Supplemental Appropriations Act (1st Act: March 6th, 2020):
March 13, 2020
Written by Stephan Peron, Patrick Speights and Kelly Titus
General Overview
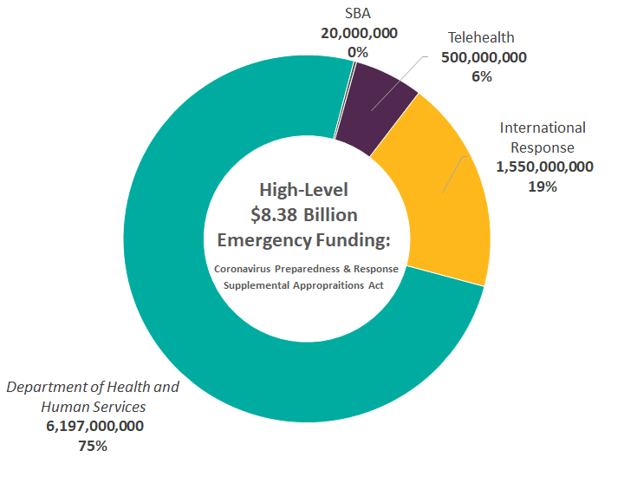
The first major legislation enacted in response to COVID-19 was the Coronavirus Preparedness and Response Supplemental Appropriations Act (“1st Act”) which was signed into law on March 6th, 2020. The bill funds various government agencies and programs to assist in the preliminary relief efforts of the COVID-19 outbreak.
Domestic Efforts ($6.7 Billion)
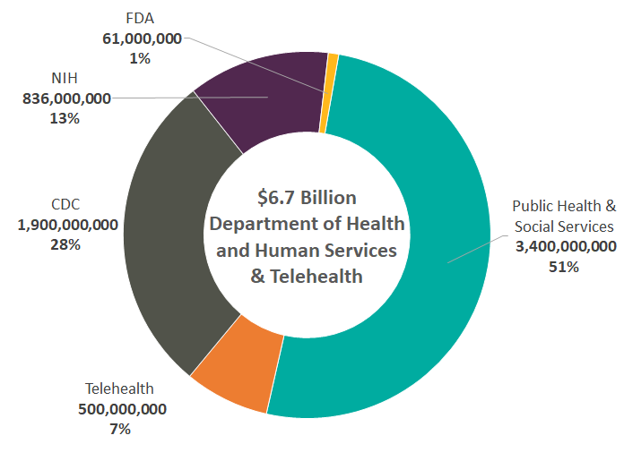
As seen above, $6.7 billion of the total $8.38 billion granted in the 1st Act are funds allocated to domestic efforts. The breakdown of funding is as follows:
Vaccine Funding: $3.4 billion for Public Health and Social Services which includes:
-
- $2 billion focused on the research and development of vaccines
- $300 million for the purchase of vaccines
- $100 million for the improvement of healthcare to geographically isolated and economically or medically vulnerable individuals
CDC Funding: $1.9 billion for the CDC which includes:
-
- $950 million for state and local response efforts
- $300 million for the replenishment of the Infectious Diseases Rapid Response Reserve Fund to help support the efforts to respond to infectious disease emergencies
NIH Funding: $0.8 billion for the National Institute of Allergy and Infectious Diseases (NIAID) to conduct research on therapies, vaccines, diagnostics, and other health technologies at the NIH
FDA Funding: $61 million to the FDA for the development and review of vaccines, therapeutics, medical devices and countermeasures, address potential supply chain interruptions, and the enforcement of counterfeit products
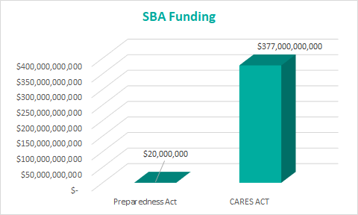
SBA Loans: $20 million for SBA disaster loans that will be made available to entities financially impacted due to COVID-19
(Provided is a visual aid in the drastic addition to SBA funding from the March 6th Act to the March 27th Act. The Preparedness Act is equivalent to 0.0053% of the amount awarded in the CARES Act.)
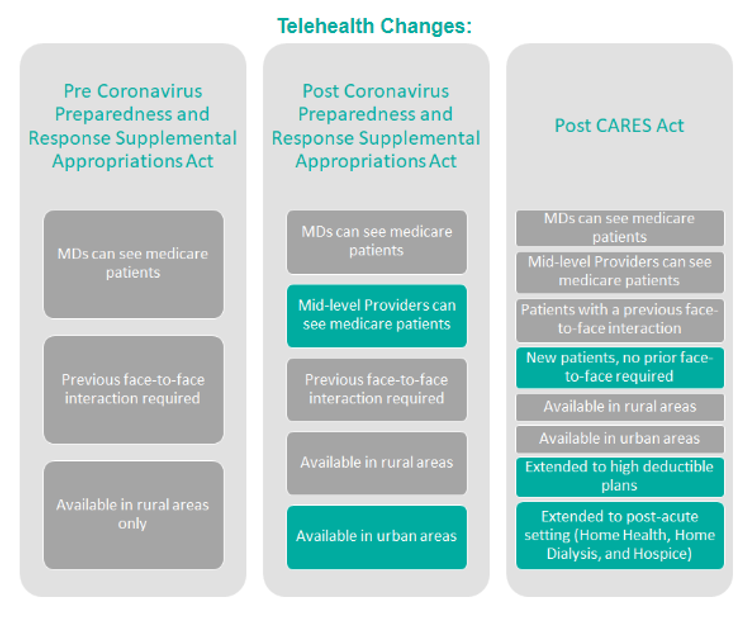
Telehealth: At an estimated cost of $500 million, the bill includes a waiver removing restrictions on Medicare providers allowing them to offer telehealth services regardless of whether the beneficiary of these services is in a rural community
International Efforts ($1.6 billion)
- Global Health Program: $435 million for the Global Health Program to support health systems responding to COVID-19 abroad.
- International Disaster Assistance: $300 million for the International Disaster Assistance to support humanitarian needs due to COVID-19.
- Economic Support Funds: $250 million for Economic Support Funds to support economic security and stability efforts in response to COVID-19.
- U.S. Embassies: $264 million to support the needs of U.S. embassies, such as emergency evacuations, during COVID-19.
CDC Funding: $300 million to support the CDC’s global disease detection and response efforts.
Sources
- https://www.congress.gov/116/bills/hr6074/BILLS-116hr6074enr.pdf
- https://www.congress.gov/bill/116th-congress/house-bill/6074
- https://www.kff.org/global-health-policy/issue-brief/the-u-s-response-to-coronavirus-summary-of-the-coronavirus-preparedness-and-response-supplemental-appropriations-act-2020/
- https://www.congress.gov/bill/116th-congress/house-bill/6201
- https://www.congress.gov/bill/116th-congress/house-bill/6201/text
- https://appropriations.house.gov/sites/democrats.appropriations.house.gov/files/Families%20First%20summary.pdf
- https://www.kff.org/global-health-policy/issue-brief/the-families-first-coronavirus-response-act-summary-of-key-provisions/
- https://www.dol.gov/agencies/whd/pandemic/ffcra-employee-paid-leave#_ftnref3
- https://www.councilofnonprofits.org/trends-policy-issues/what-the-families-first-coronavirus-response-act-means-nonprofits
- https://www.chapman.com/insights-publications-CARES_Act_Health_Care_Businesses.html
Categories:
Subscribe
to our blog